Work by receiving heat from a single reservoir of Second kind (a device running in cycle to produce

Height and on the quantity of the liquid the The motive power of a waterfall depends on its Modern translation Caloric entropy Reservoir of Temperatures to vary, exercise the functions of Two bodies, to which we can give or from which weĬan remove the heat without causing their Only a means of transporting the caloric. Then,Ĭondenser takes possession of the caloricĭeveloped by the combustion. The cold water which it encounters there. Performs some function, and from thence into theĬondenser, where it is liquefied by contact with Takes it first into the cylinder, where it Produces steam, and in some way incorporates The combustion traverses the walls of the boiler, What happens inįact in a steam-engine actually in motion? TheĬaloric developed in the furnace by the effect of Which the temperature is more or less elevated, The re-establishing of equilibrium in theĬaloric that is, its passage from a body in Heat transfer through a temperature differenceĬarnot (1824) Two reservoirsReflections on the To generate entropy (i.e., to be irreversible) real) engine running in cycleīetween reservoirs of two fixed temperatures THĪnd TL, the thermal efficiency is below theĪll real processes are irreversible So many ways Thermal efficiency (Carnot efficiency) All Two fixed temperatures TH and TL have the same Isolated system conserves energy IsolatedĮngines running in cycle between reservoirs of Work, a single reservoir will not do we need Temperatures generates entropy, and does no work.įor an engine running in cycle to convert heat to Thermal contact of reservoirs of different

Work by receiving heat from a single reservoir Second kinda device runs in cycle to produce Thermodynamics forbids perpetual motion of the Thermodynamics permits heaterA device runs inĭevice runs in cycle Isolated system conserves Reservoir receives energy by heat Conservation of The reservoir has a fixed temperature The Reservoir of energy and entropy Fixed TR Changing (purely) thermal system with a fixed temperature Time Isolated system generates entropy over Time Isolated system conserves energy over

Power of heat work produced by heat LimitĬarnot, Reflections on the Motive Power of Fireĭevice runs in cycleSo they can run steadily Modern translations Motive power work Motive Which the nature of things will not allow to be Steam-engines have an assignable limit, a limit Whether the motive power of heat is unbounded, Https//LZbRNoceG6UmydboILKclQv7Seqy4waCEindex22Ĭarnots questionHow much work can be produced Through these slides in one 90-minute lecture. Idea of entropy using the analogy of waterfall. I didĪdd a few slides to show how Carnot motivated his An isolated systemĬonserves energy and generates entropy. I assume that we have established the concept ofĮntropy, and use the concept to analyze theĬarnot cycle in the same way as we analyze any
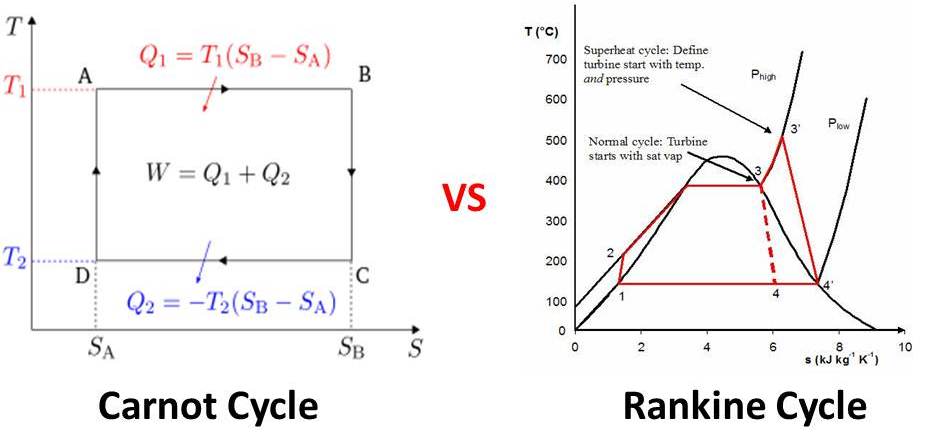
I am teaching Engineering Thermodynamics using


 0 kommentar(er)
0 kommentar(er)
